3D illustration of two molecular structures, (S)-MA and (R)-MA, with OH labels, connected to a larger blue and silver cage molecule, on a scientific data background.
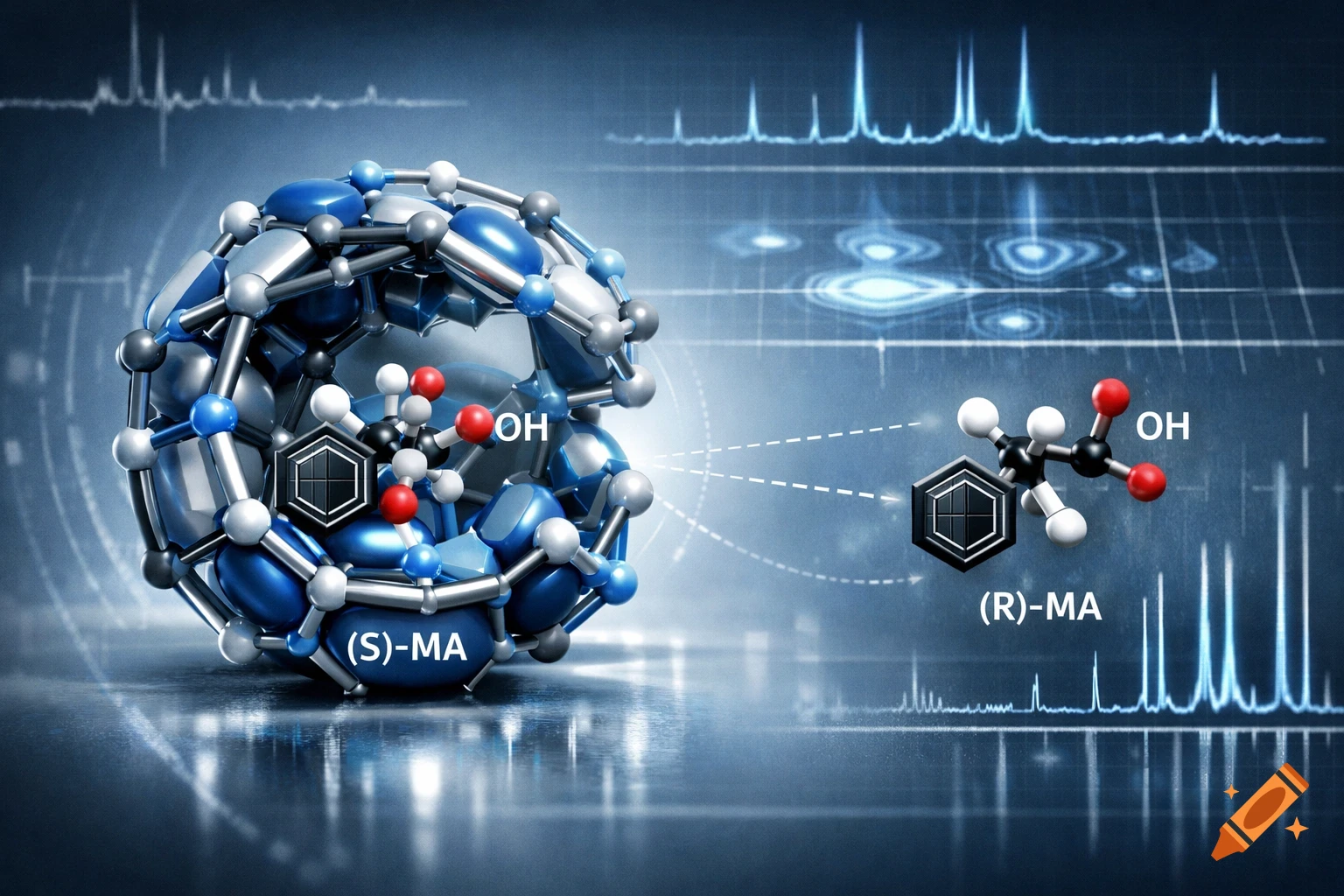
The differentiation and assignment of absolute configurations of enantiomers in mixtures remains a significant challenge for both experimental and computational methods. In recent years, diffusion-NMR experiments using chiral resolving agents have emerged as a promising approach for enantiodiscrimination. While many resolving agents demonstrate strong enantioselective capabilities, limitations persist, particularly in reliably determining absolute configurations of the stereoselective recognition and the molecular basis for such a chiral recognition process. In this study, we combine experimental NMR (ROESY and DOSY) measurements with computational studies to investigate the enantiodiscrimination of (R,S)-Mandelic Acid (MA) enantiomers by a chiral macrocyclic (MAC). The experiments reveal a clear stereoisomeric preference of MAC for the (S)-MA enantiomer, along with a larger NMR shielding effect observed for (R)-MA. Our computational modeling indicates that these enantioselective complexation preferences arise when MAC adopts a closed conformation in complex with MA. In this state, the lowest Gibbs free energy of binding is found for the (S)-MA complex, and the calculated shielding effects are consistent with experimental NMR observations. Temperature-dependent NMR experiments further support the prevalence of the folded/closed? conformation in solution, supporting the DFT predictions. Our integrated approach reveals that the folded conformation of the resolving agent plays See more