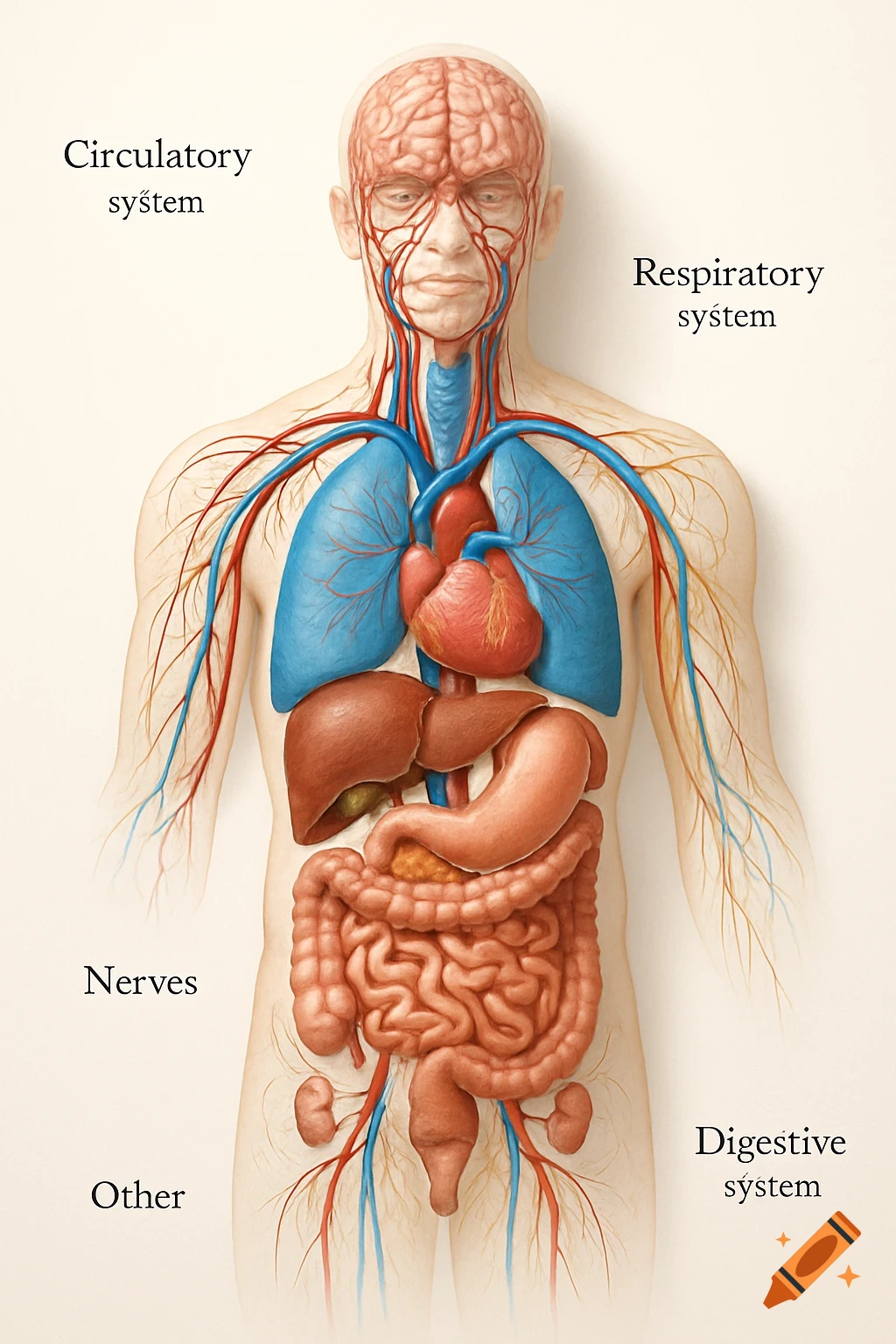
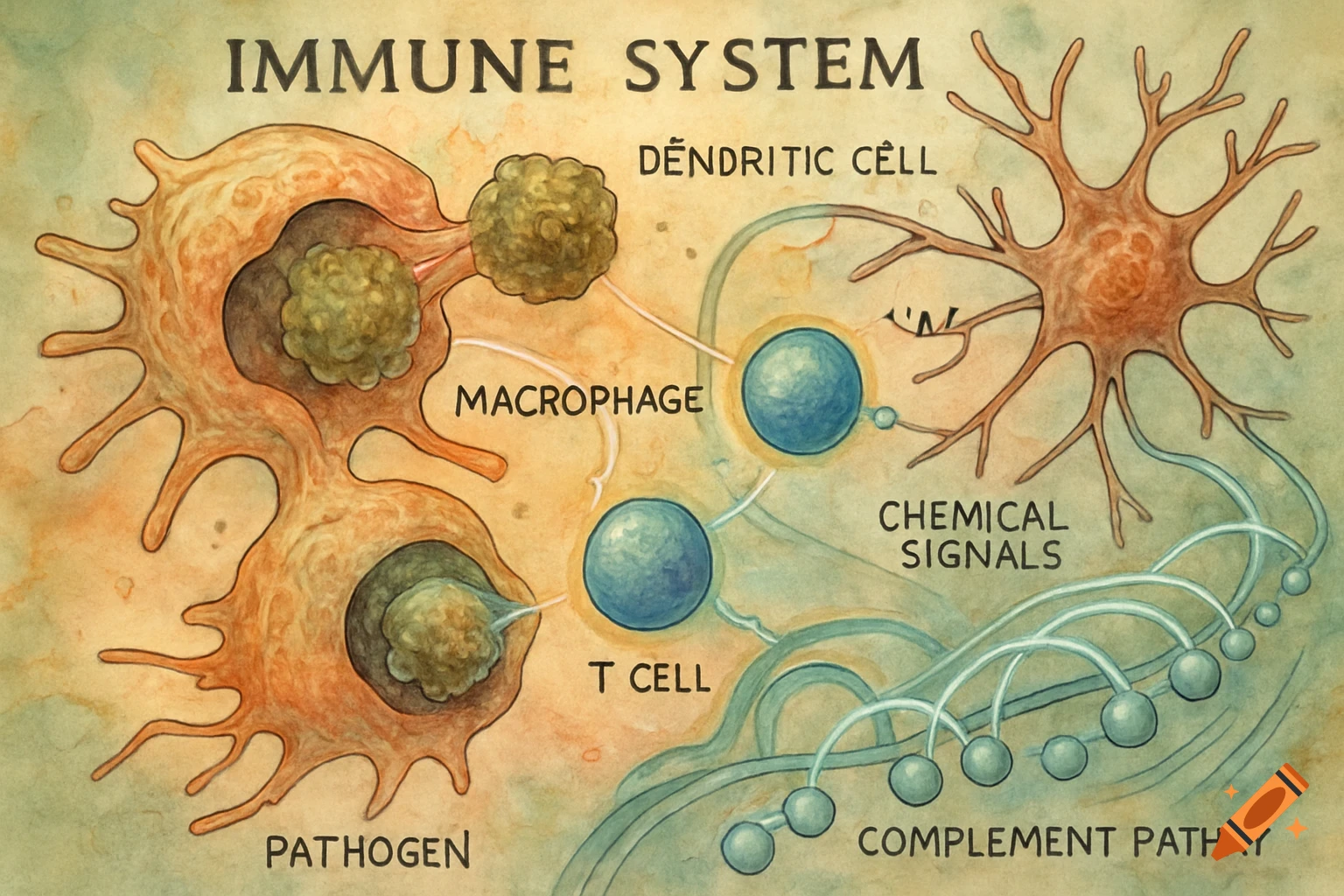
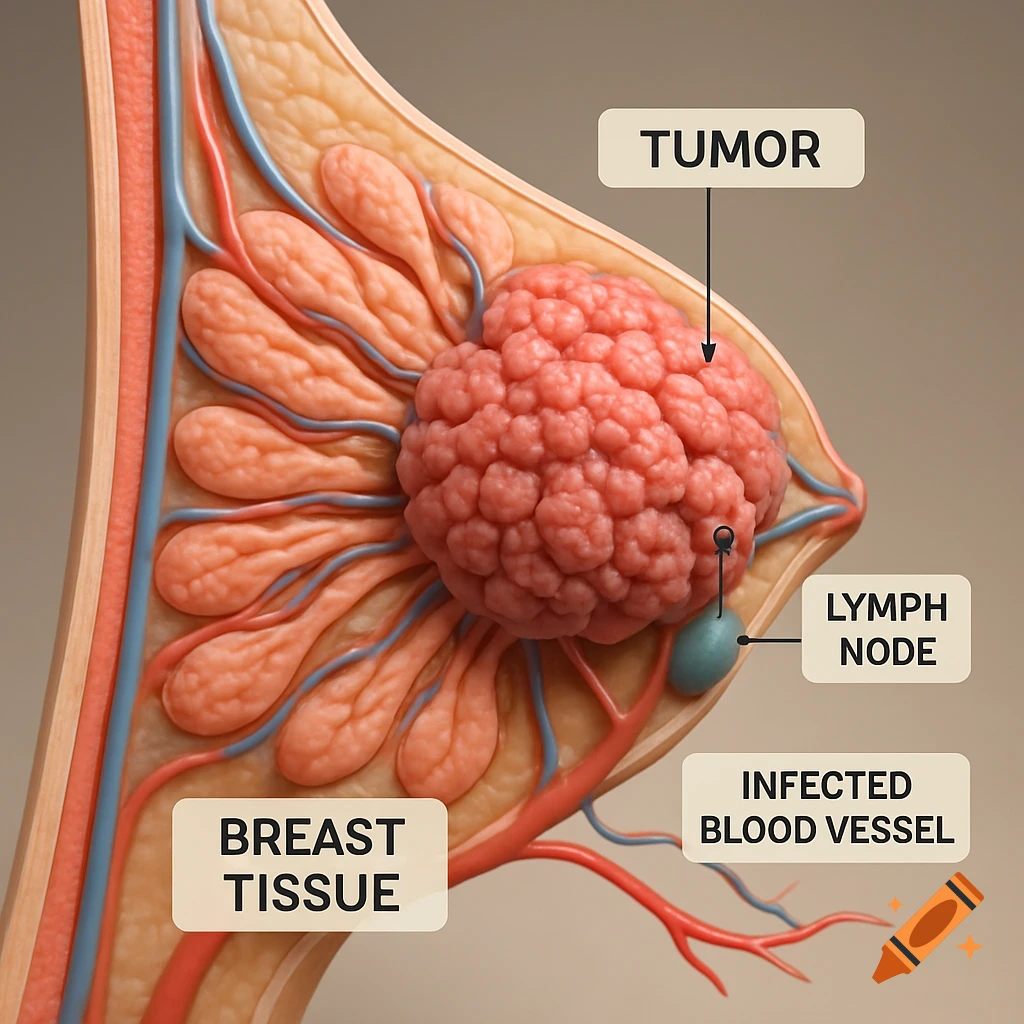
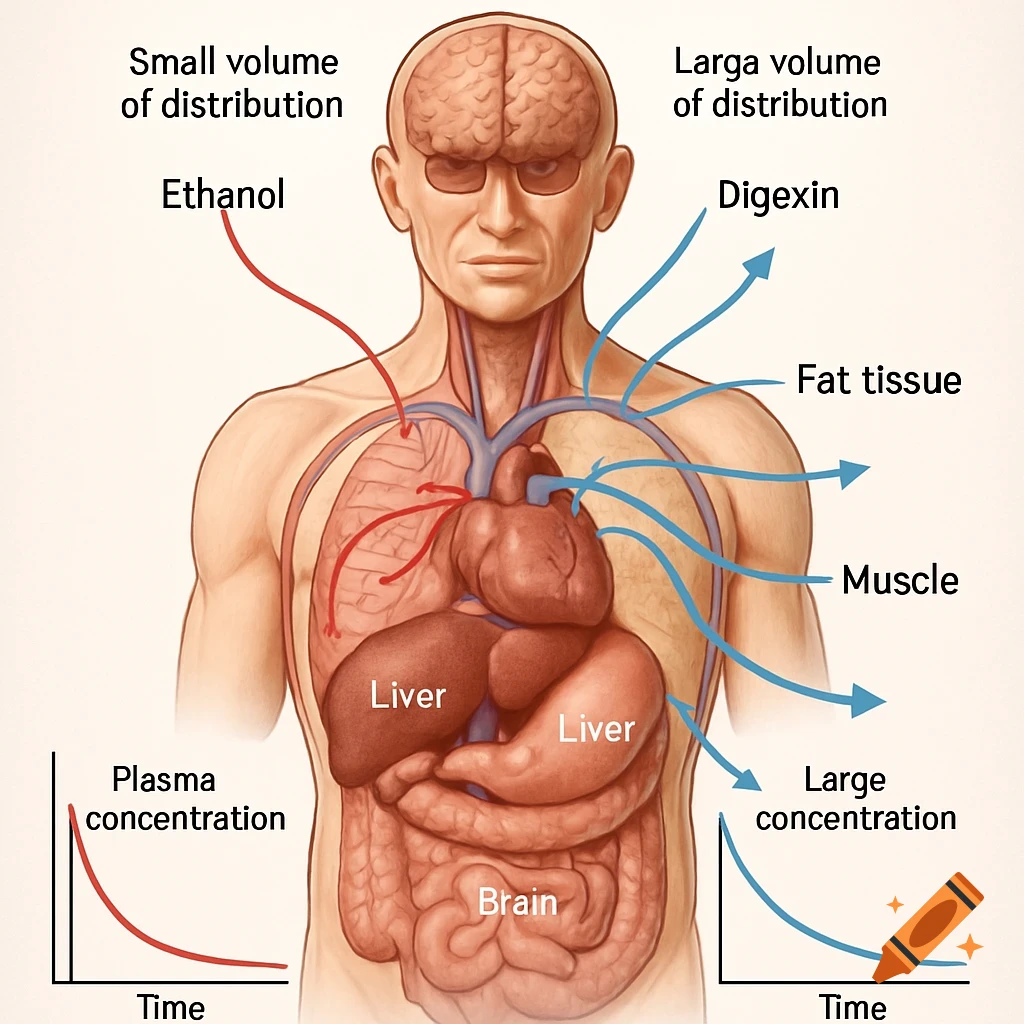
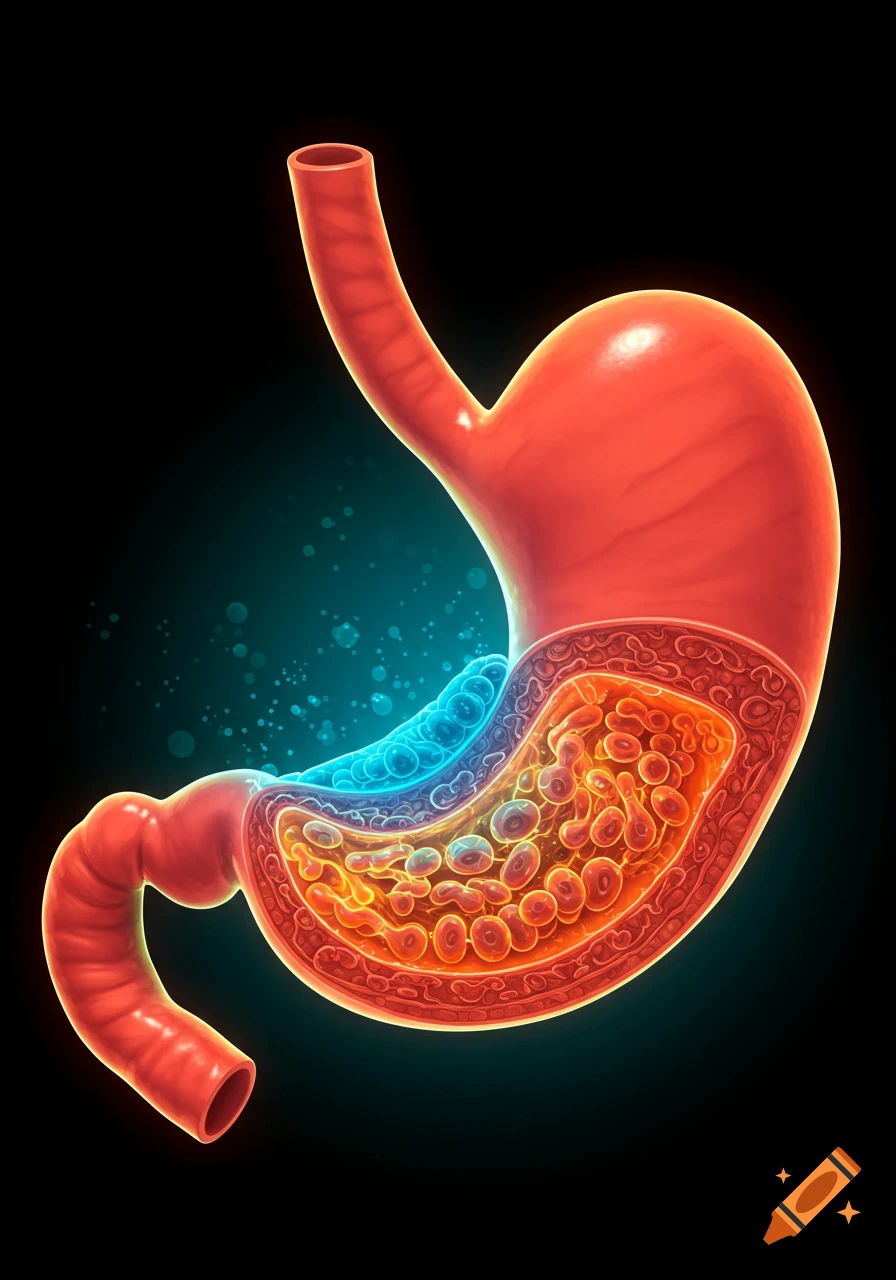
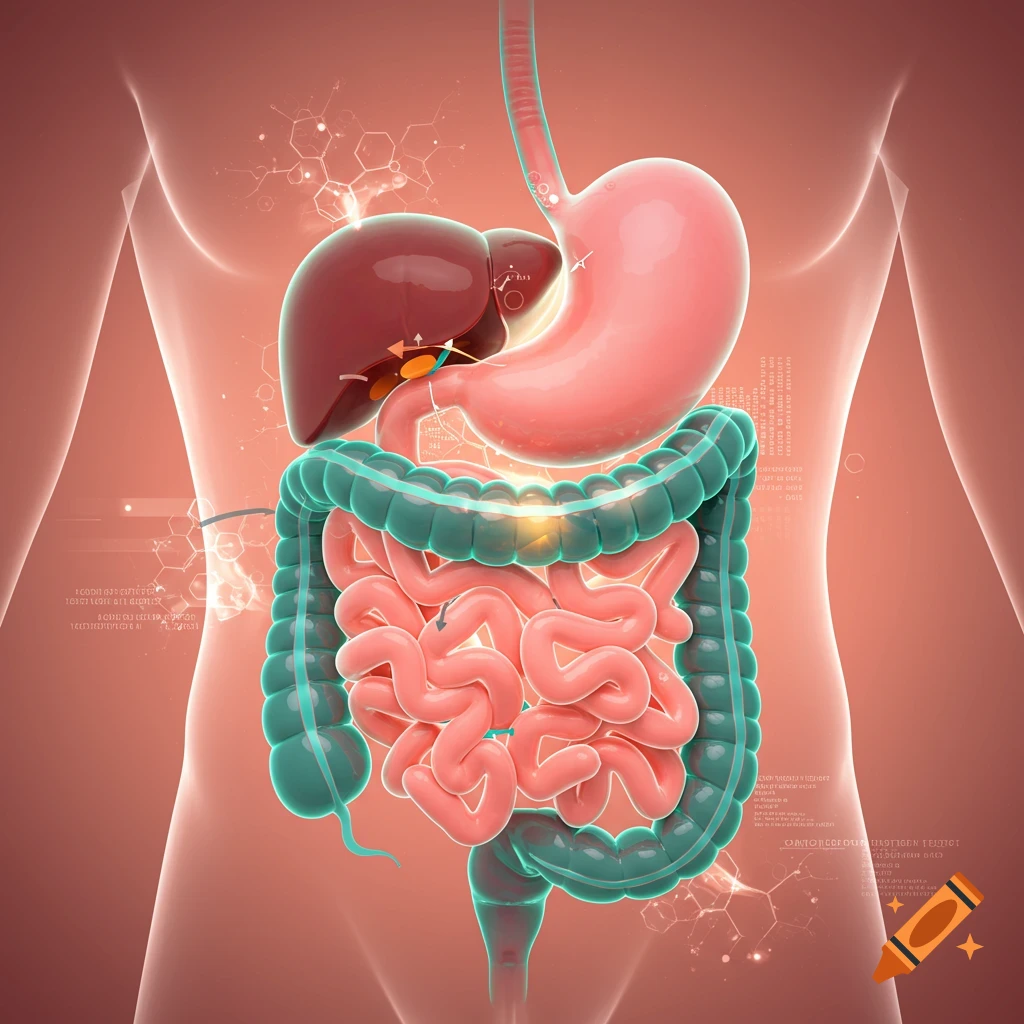
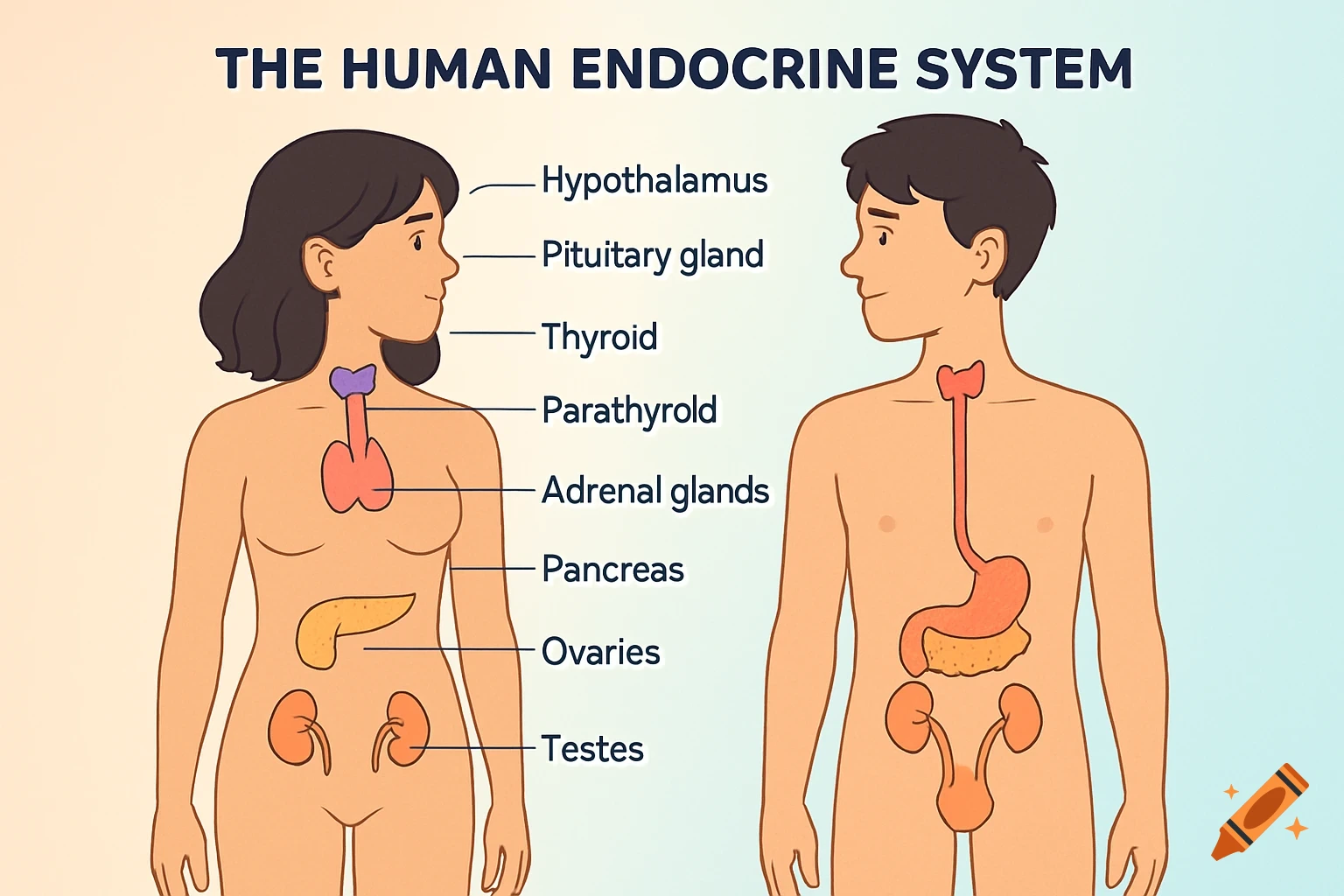
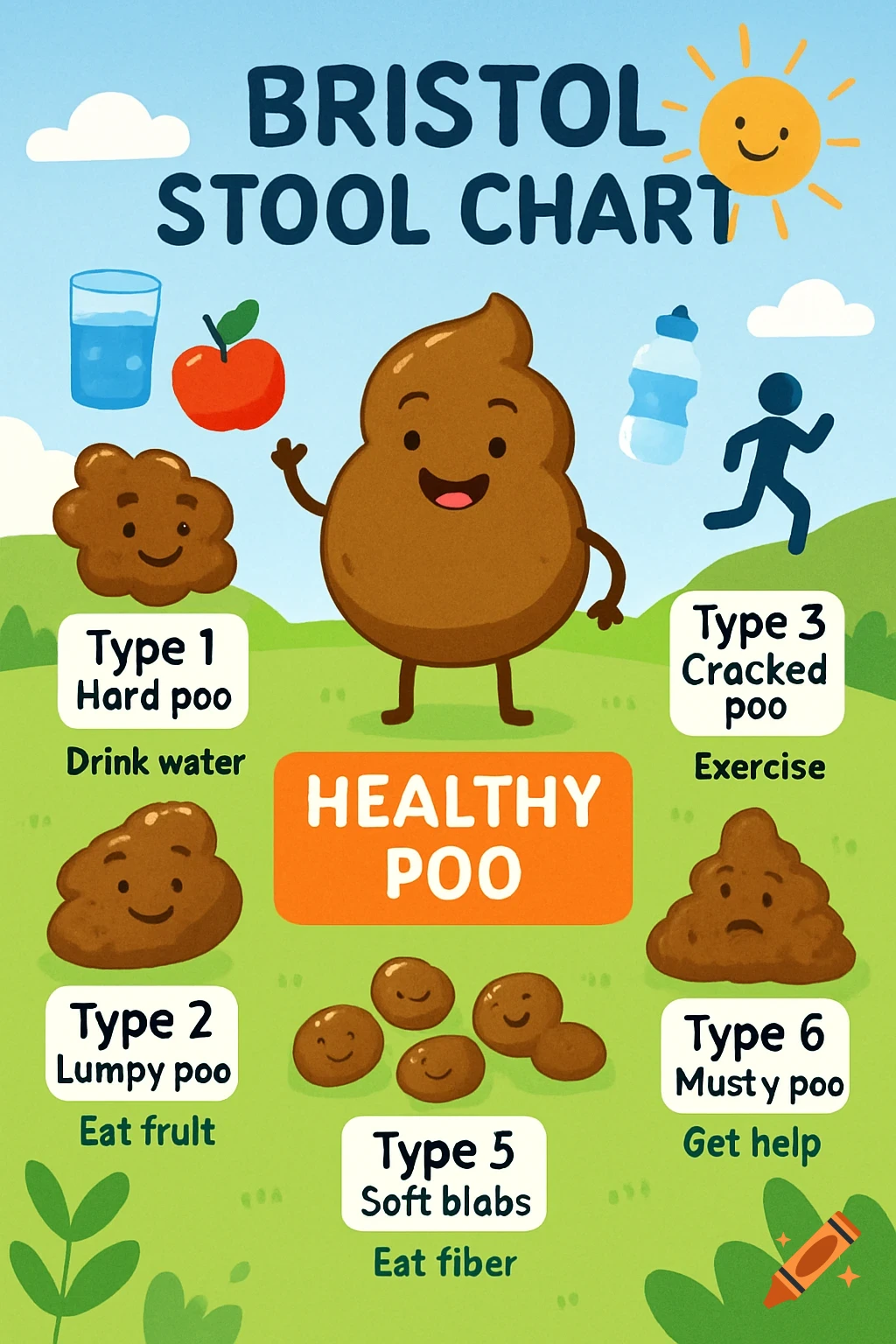
An orange-toned diagram illustrating the dietary iron absorption pathway, showing dietary iron sources, gastric acid, Dmt1, Fe2+, Fe3+, liver, macrophage, Hepcidin, Fpn, and iron storage.
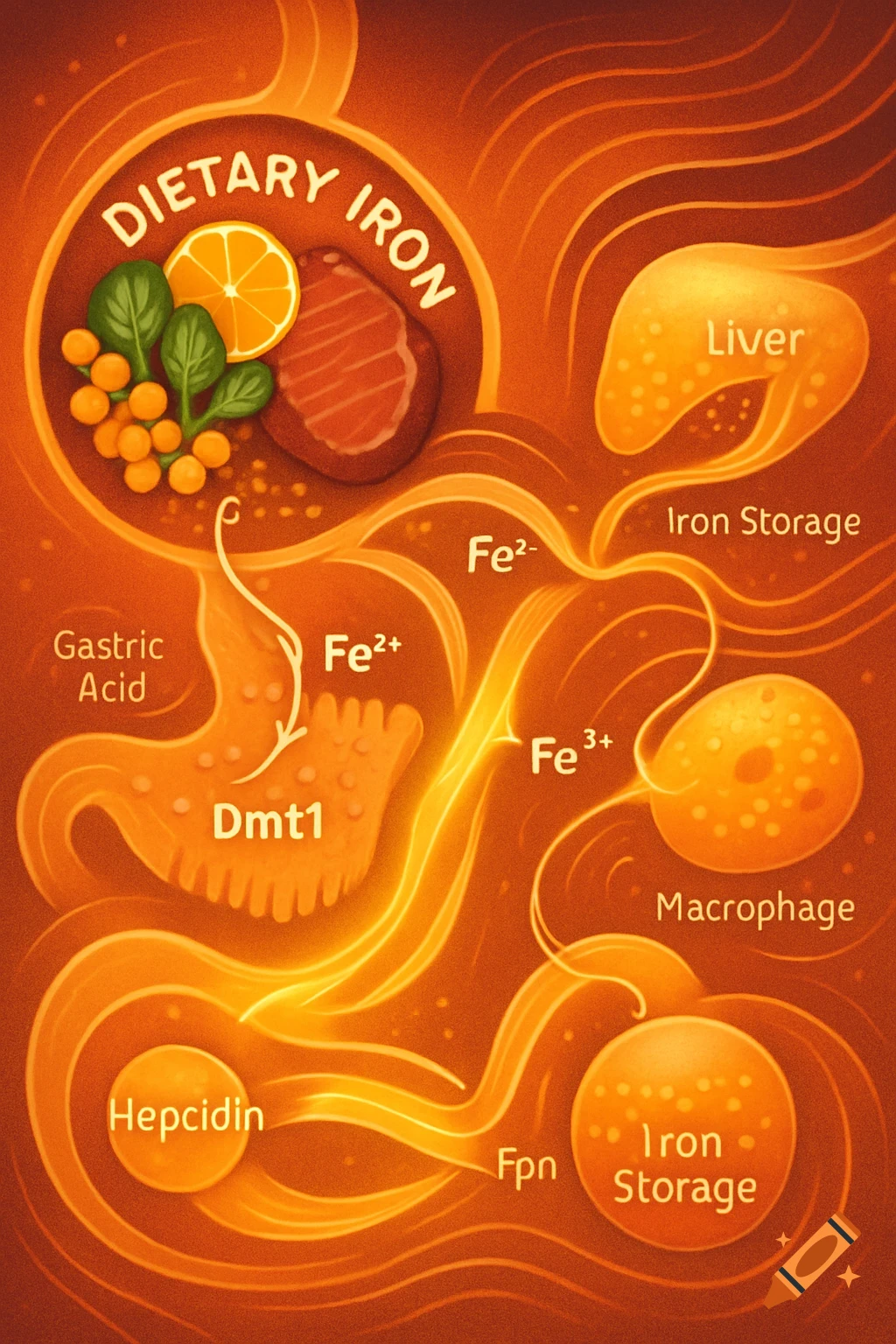
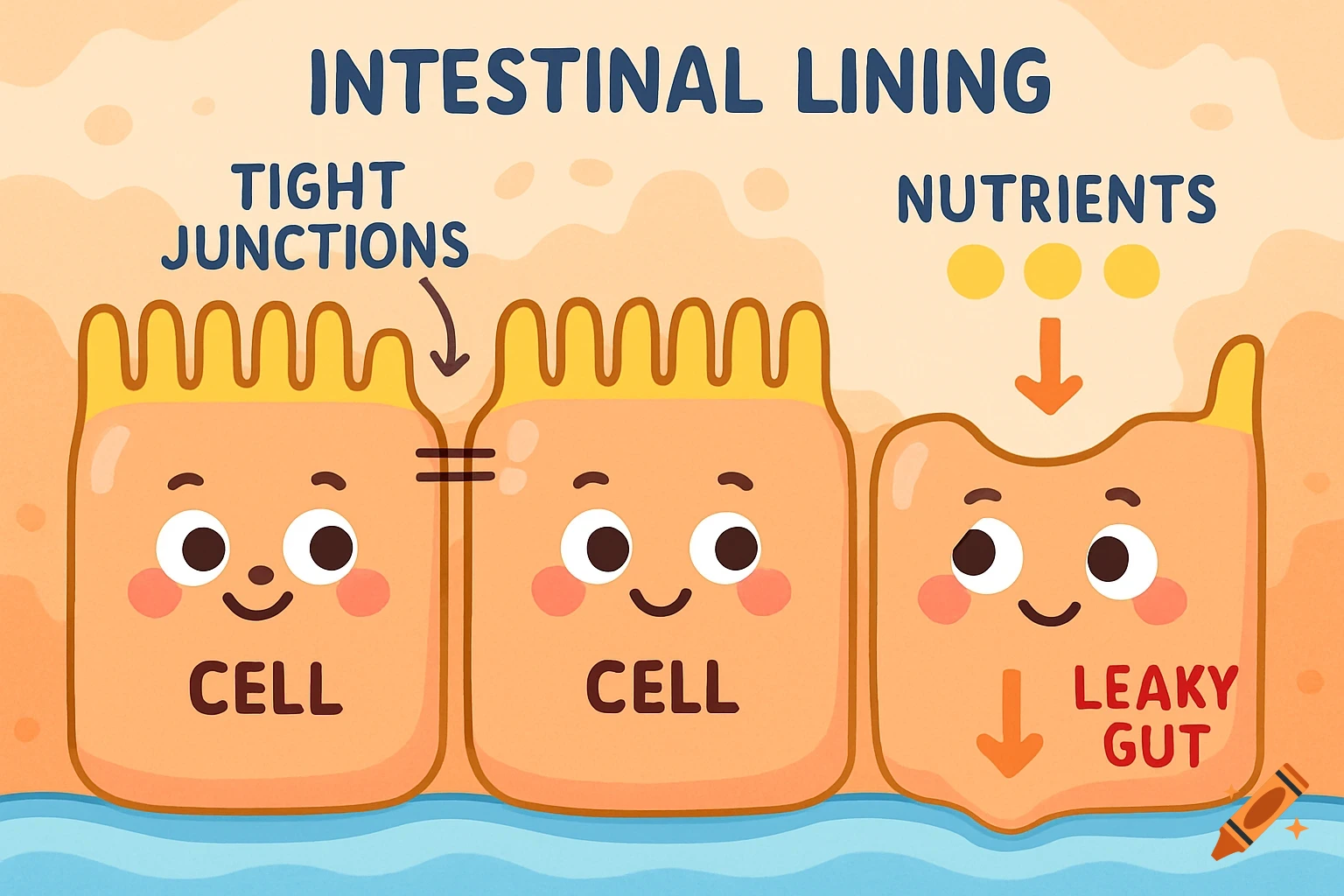
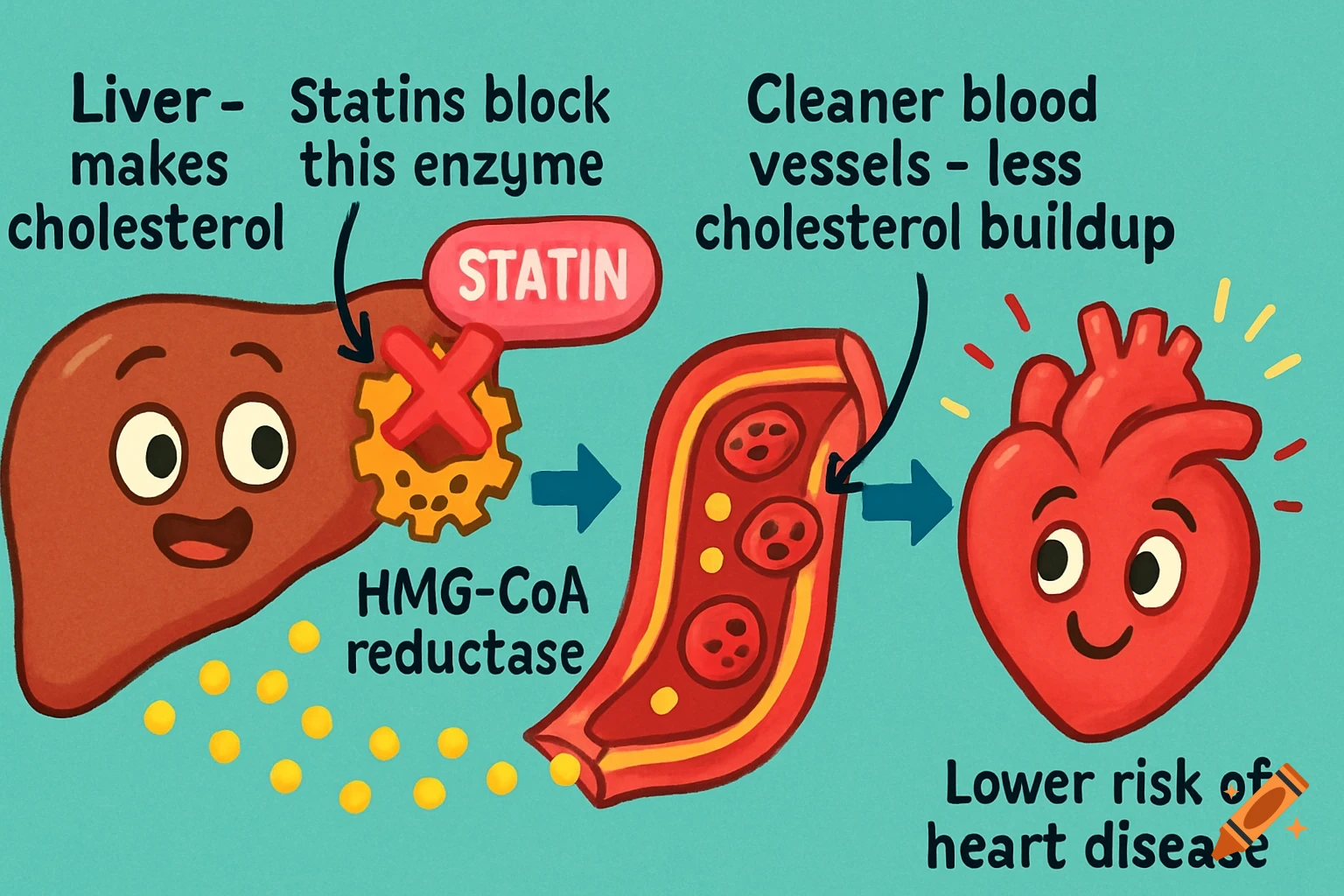
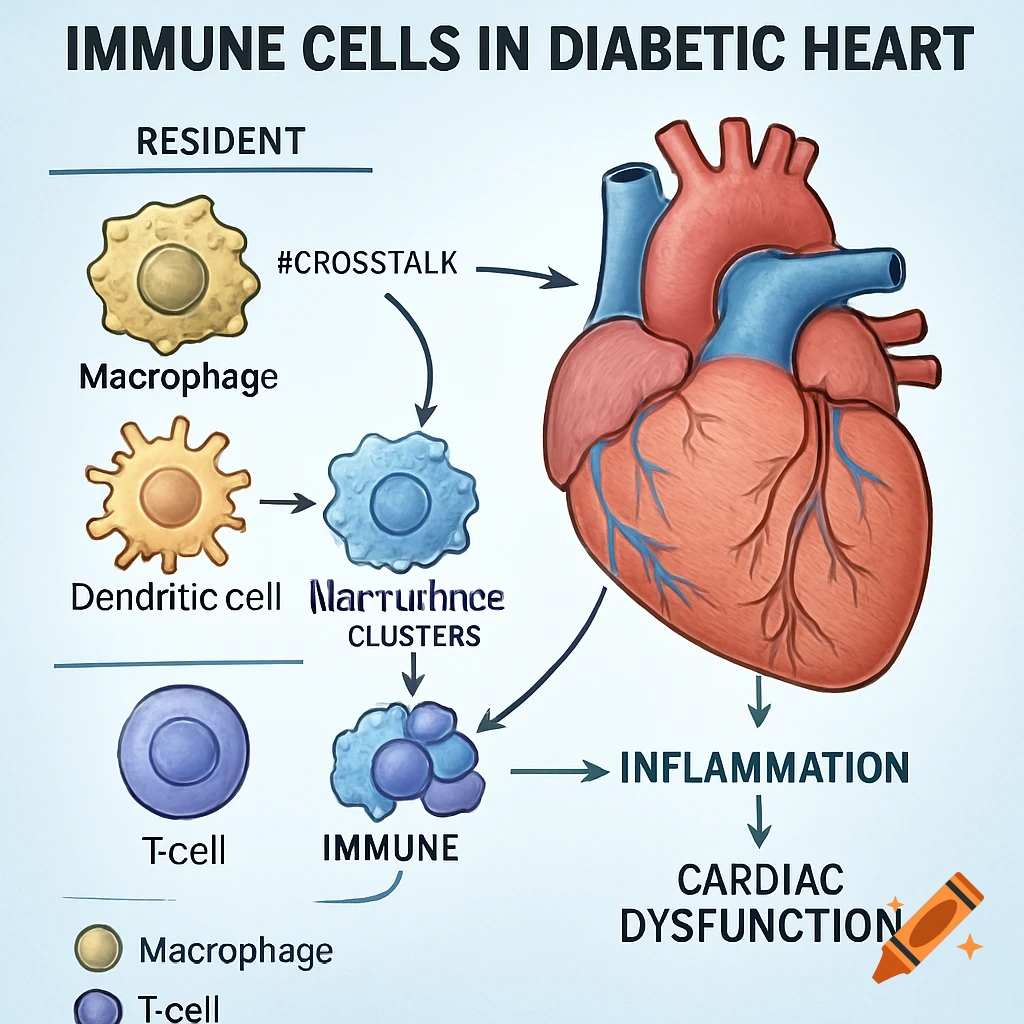
Dietary Iron Absorption:Iron from food exists mainly in two forms: heme iron (from animal sources) and non-heme iron (from plant sources).Non-heme iron (Fe^{3+}) undergoes gastric acidification in the stomach, facilitated by gastric acids, which convert Fe^{3+} to the more soluble Fe^{2+}.The enzyme Dmt1 (Divalent Metal Transporter 1) in duodenal enterocytes facilitates the uptake of Fe^{2+} into the intestinal cells.Inside the enterocyte, iron can be stored in ferritin or exported into the bloodstream.The regulation of iron absorption involves Hepcidin, a hormone that controls iron release by binding to Fpn (ferroportin), leading to its degradation and reducing iron export when iron stores are sufficient.Transport in Blood:Once in the bloodstream, Fe^{2+} is quickly oxidized back to Fe^{3+} (by enzymes like Heph – Hephaestin).Fe^{3+} is then bound to transferrin, a plasma protein responsible for transporting iron to various tissues, especially the liver, bone marrow, and other organs.Utilization:Transferrin delivers iron to cells that require it for physiological functions, such as:Hemoglobin synthesis in erythrocytes (red blood cells).Myoglobin in muscle tissues.Iron is utilized in the formation of hemoglobin, which is abundant in erythrocytes, with approximately 500 mg + 2500 mg stored in hemoglobin.Storage:Excess iron not immediately required for utilization is stored primarily in the liver and macrophages (such as those in the spleen and other organs) bound to See more