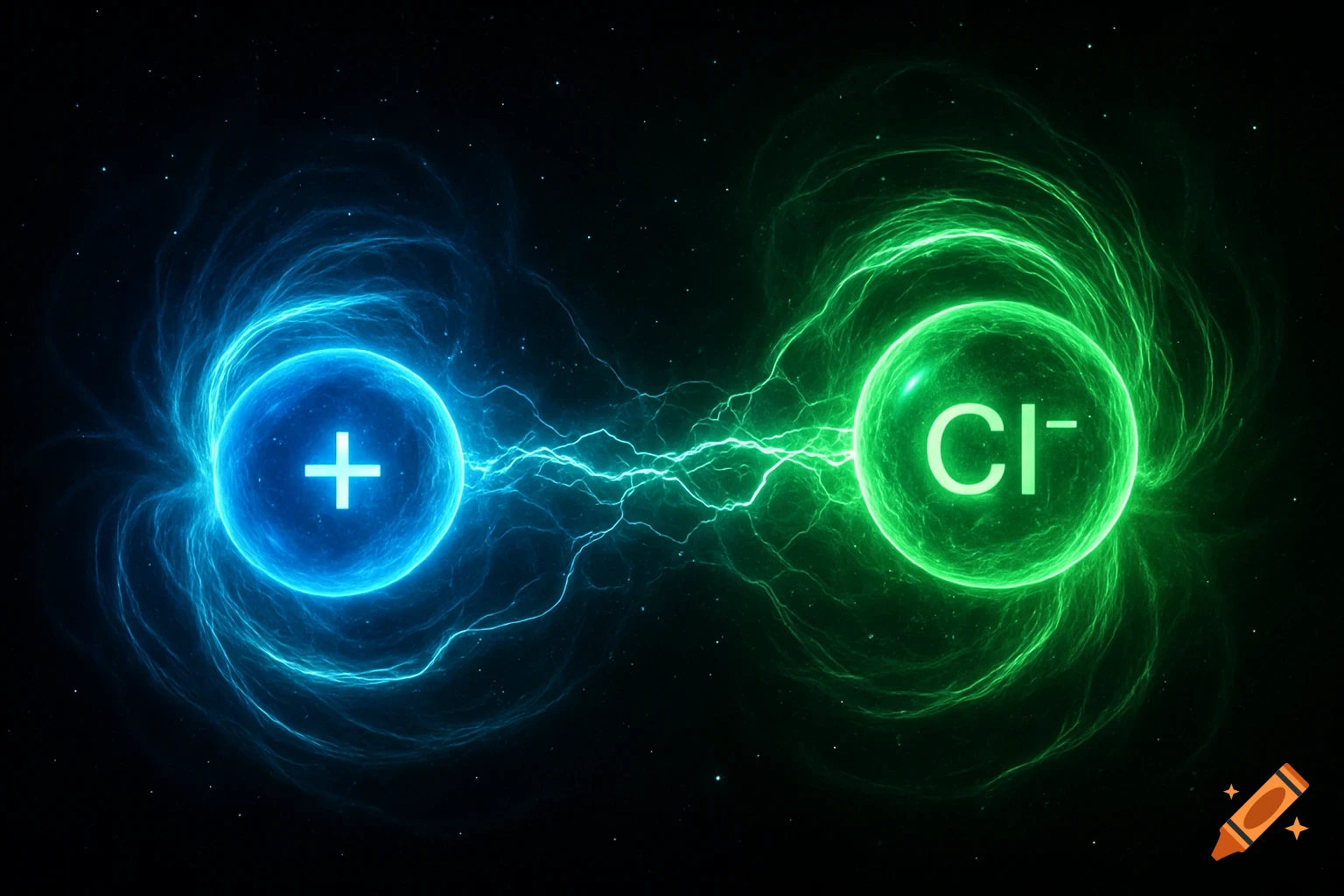
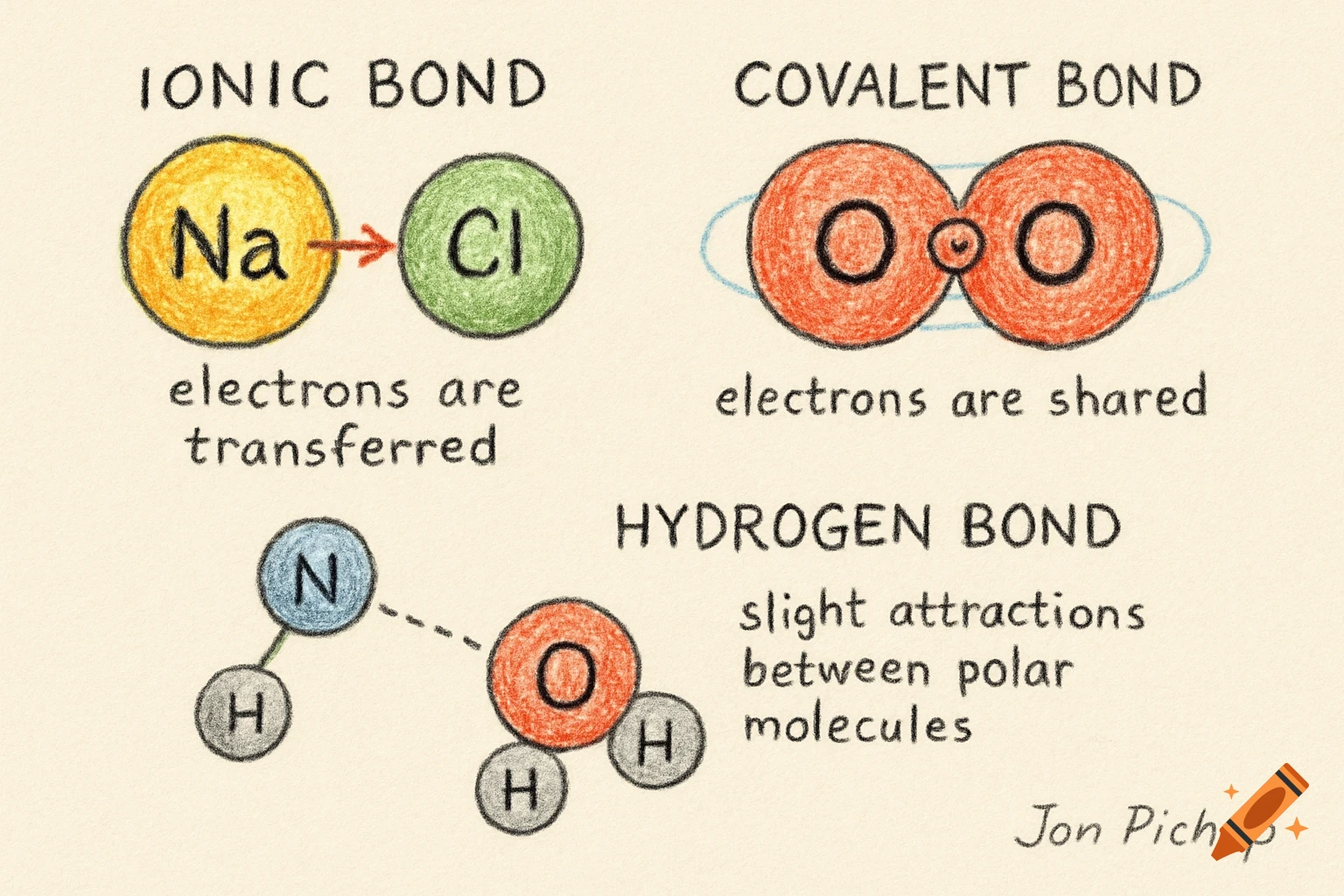
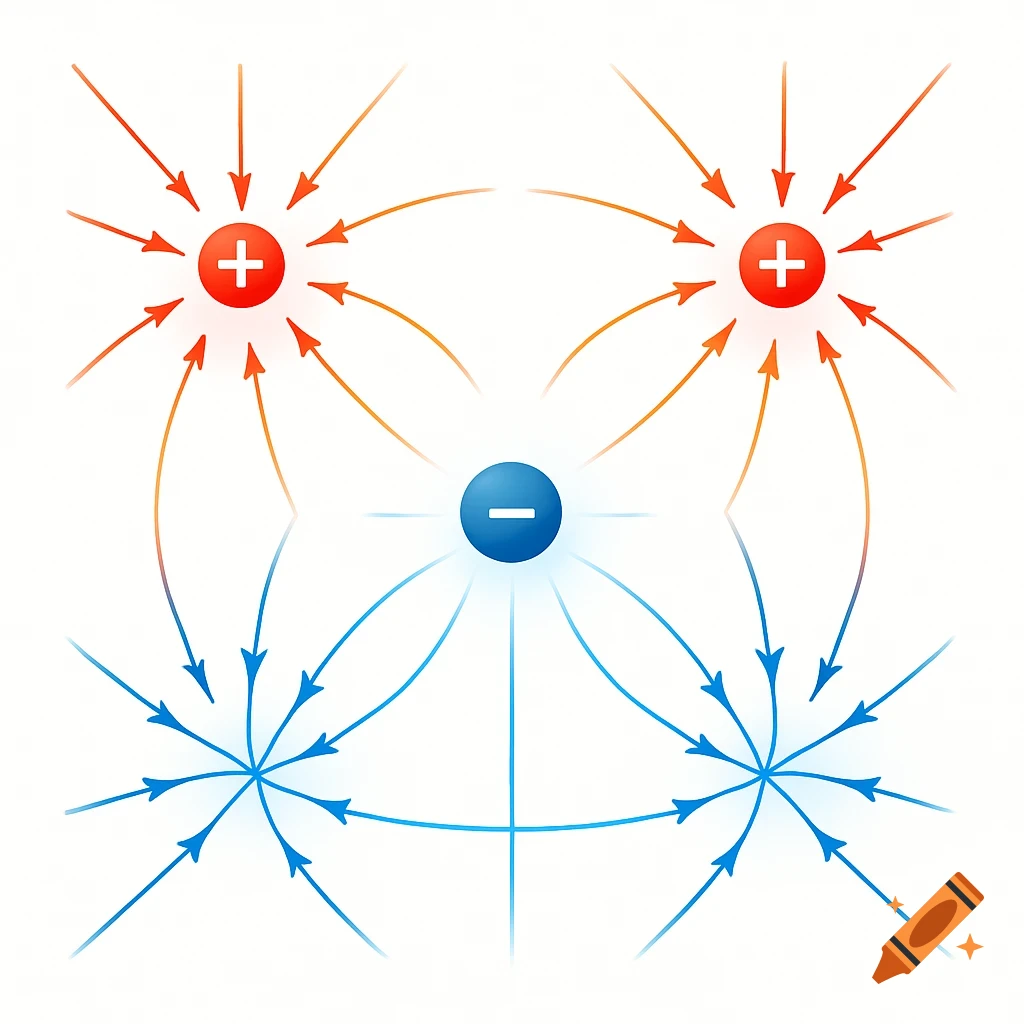
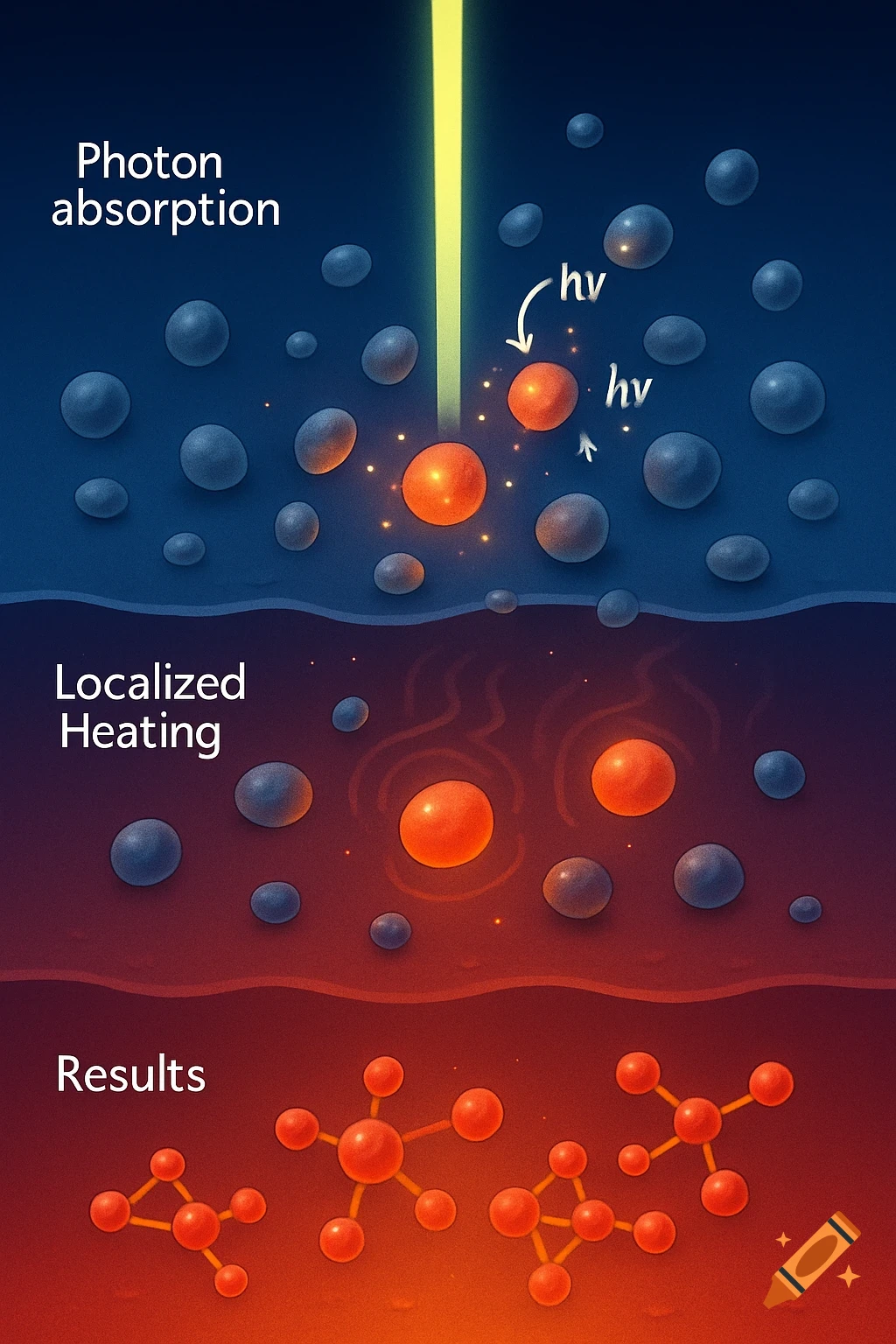
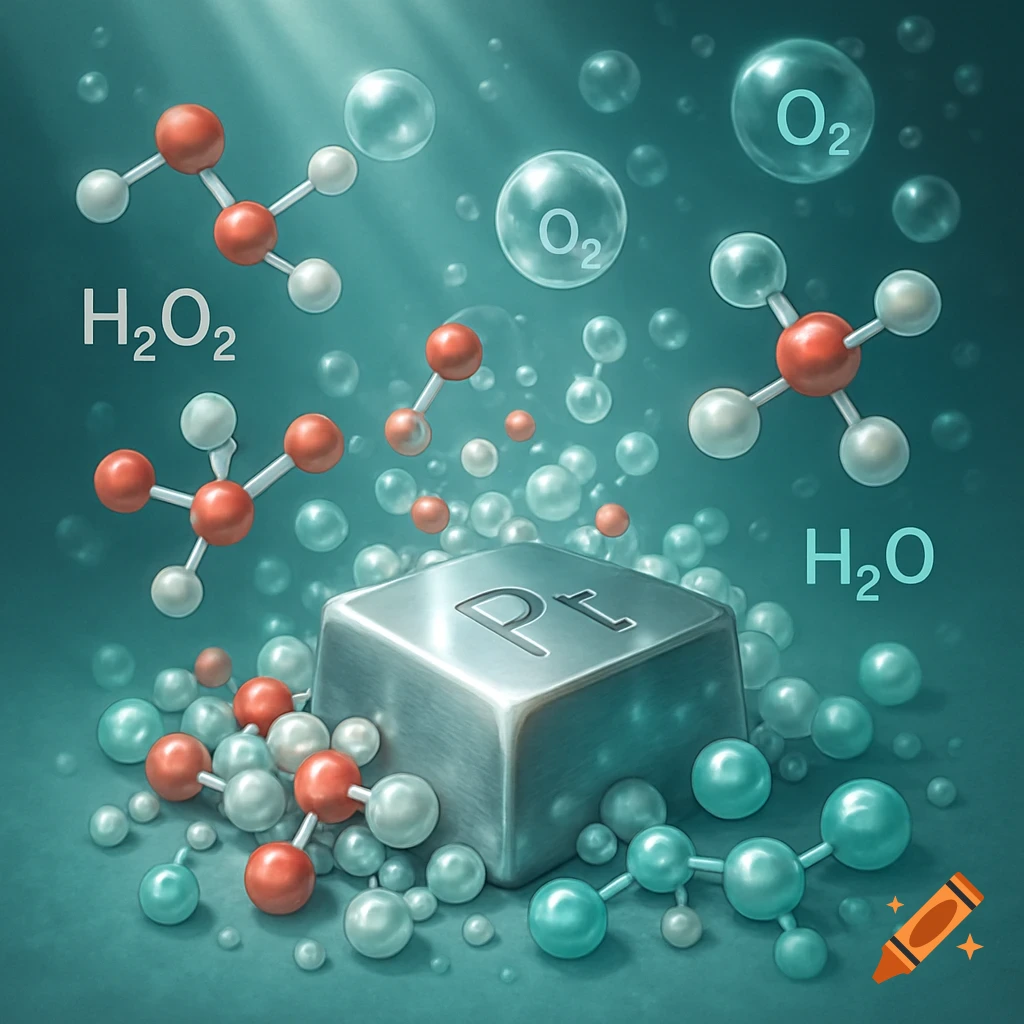
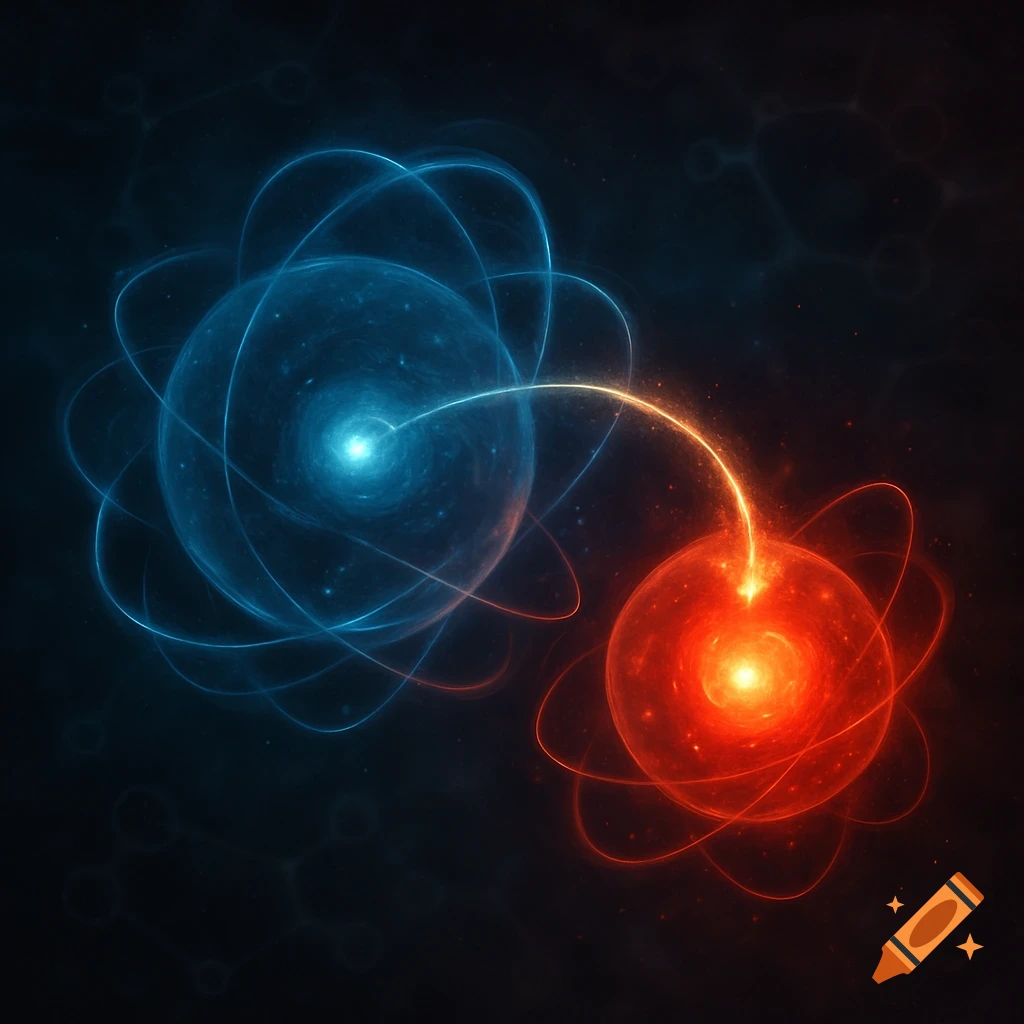
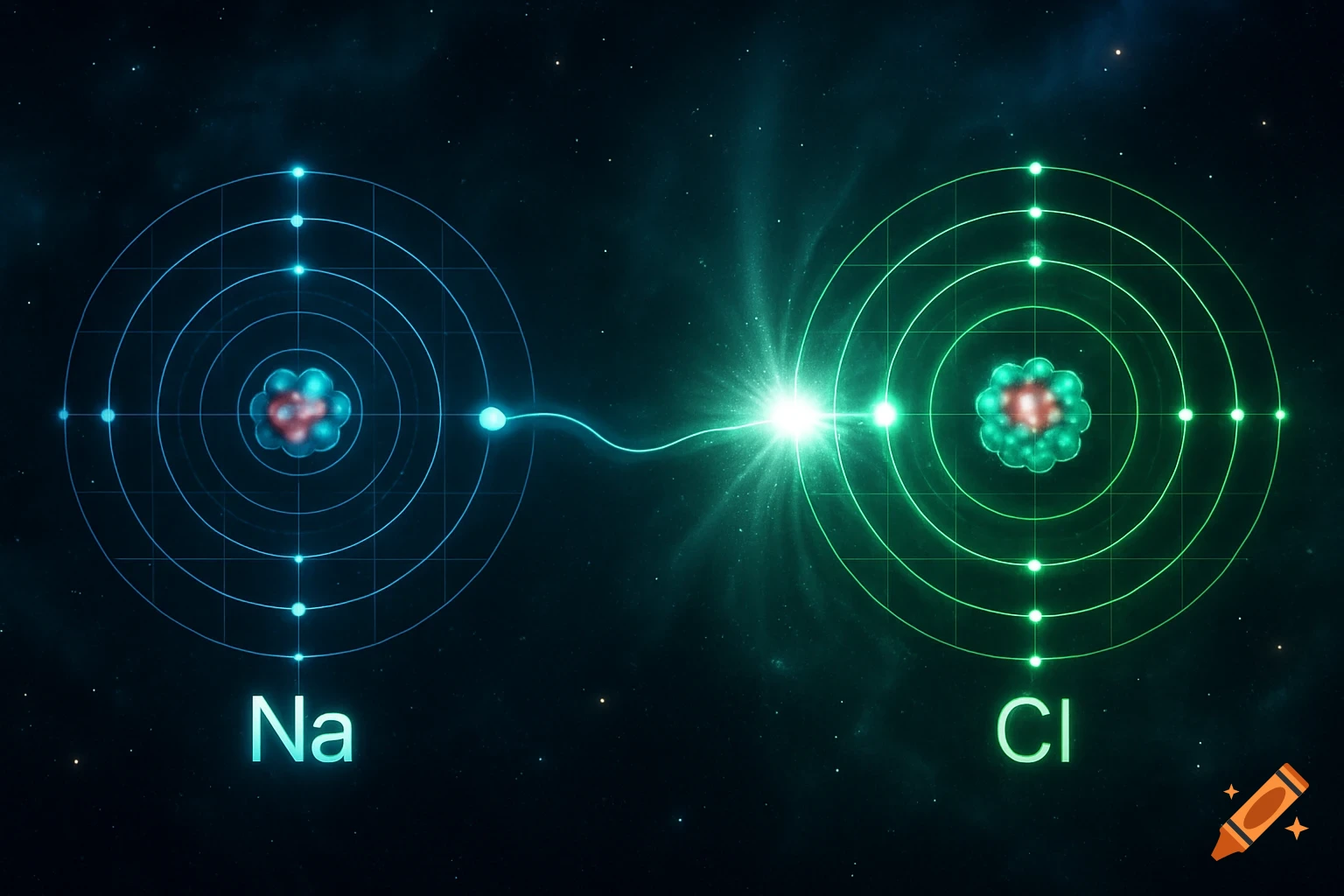
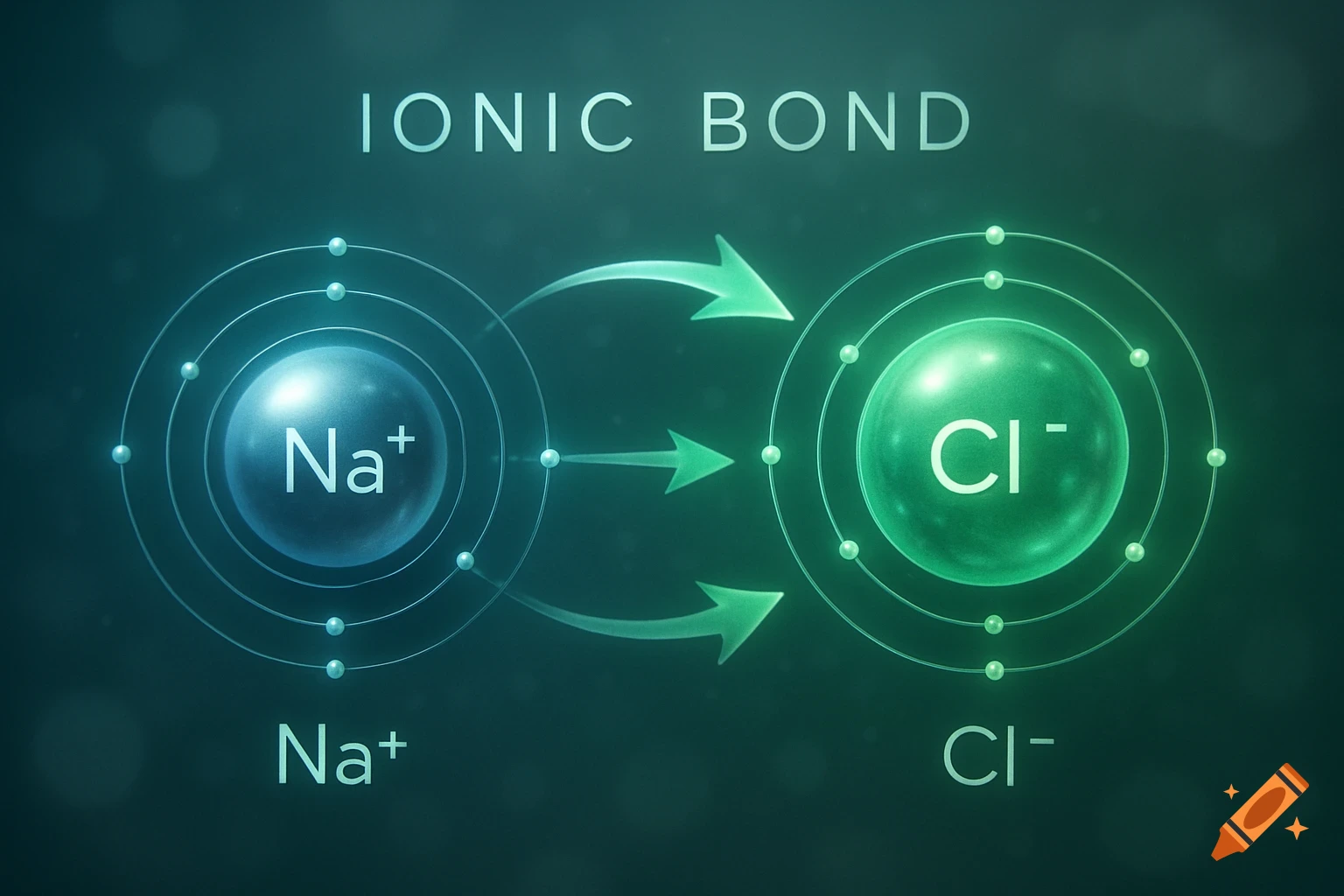
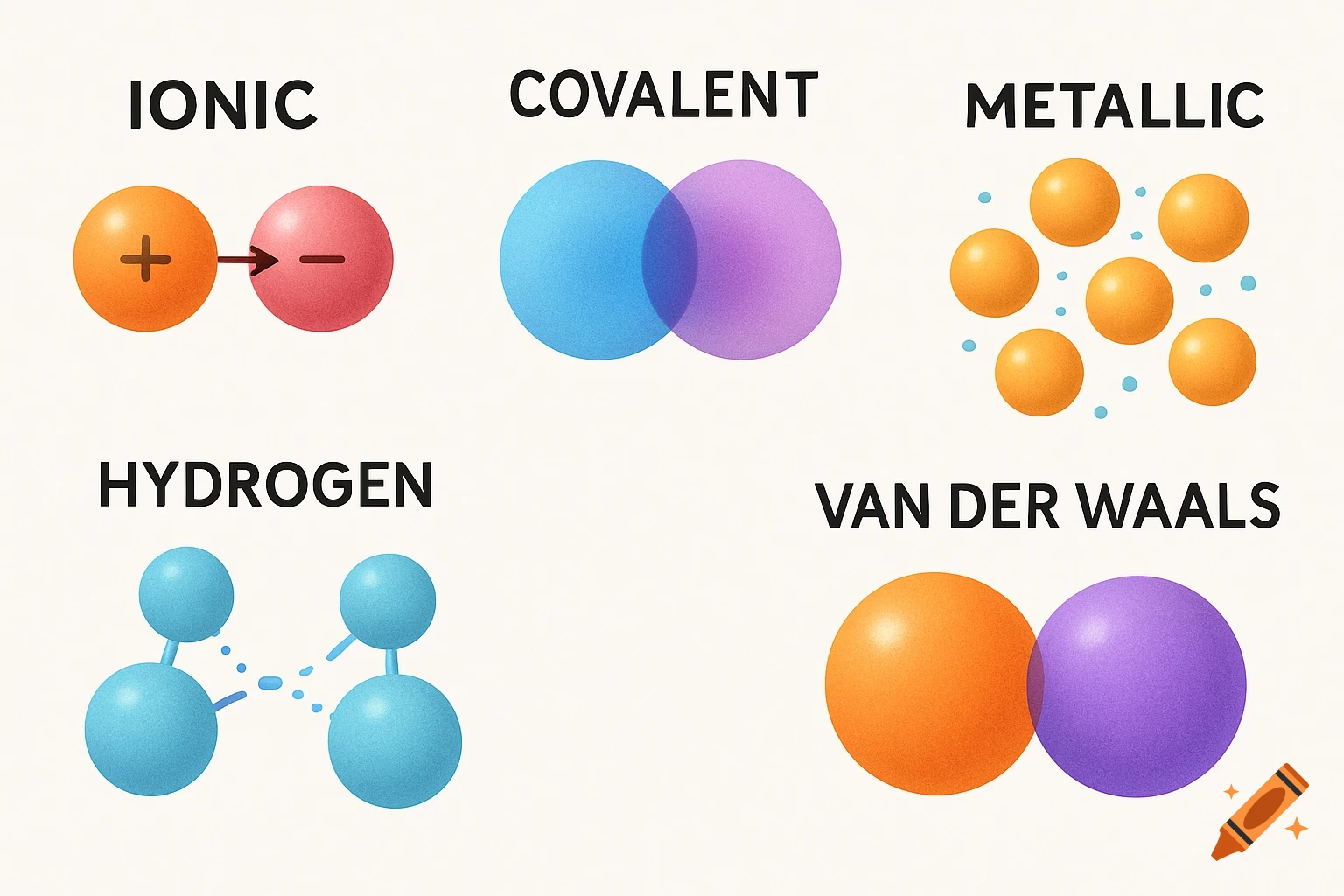
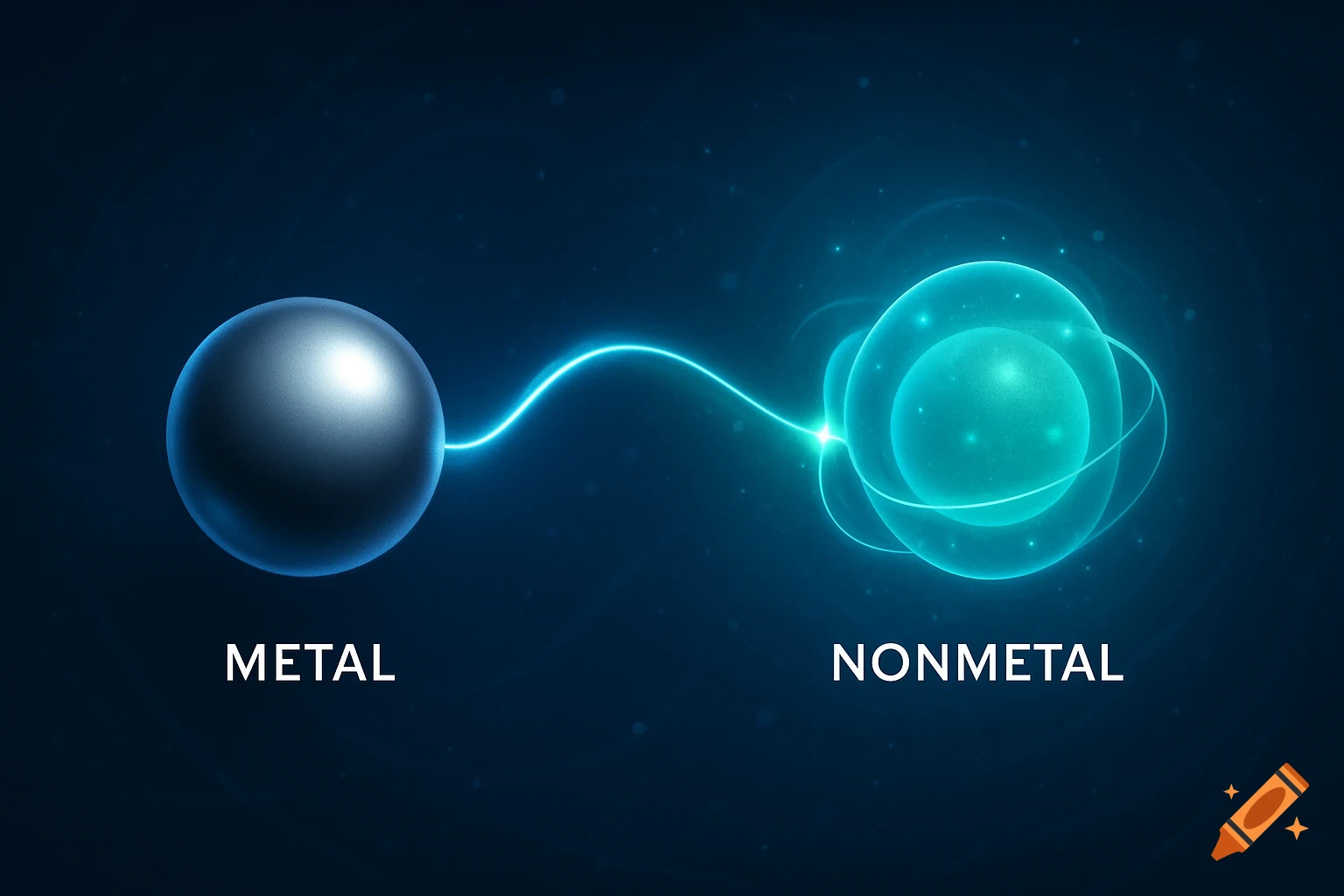
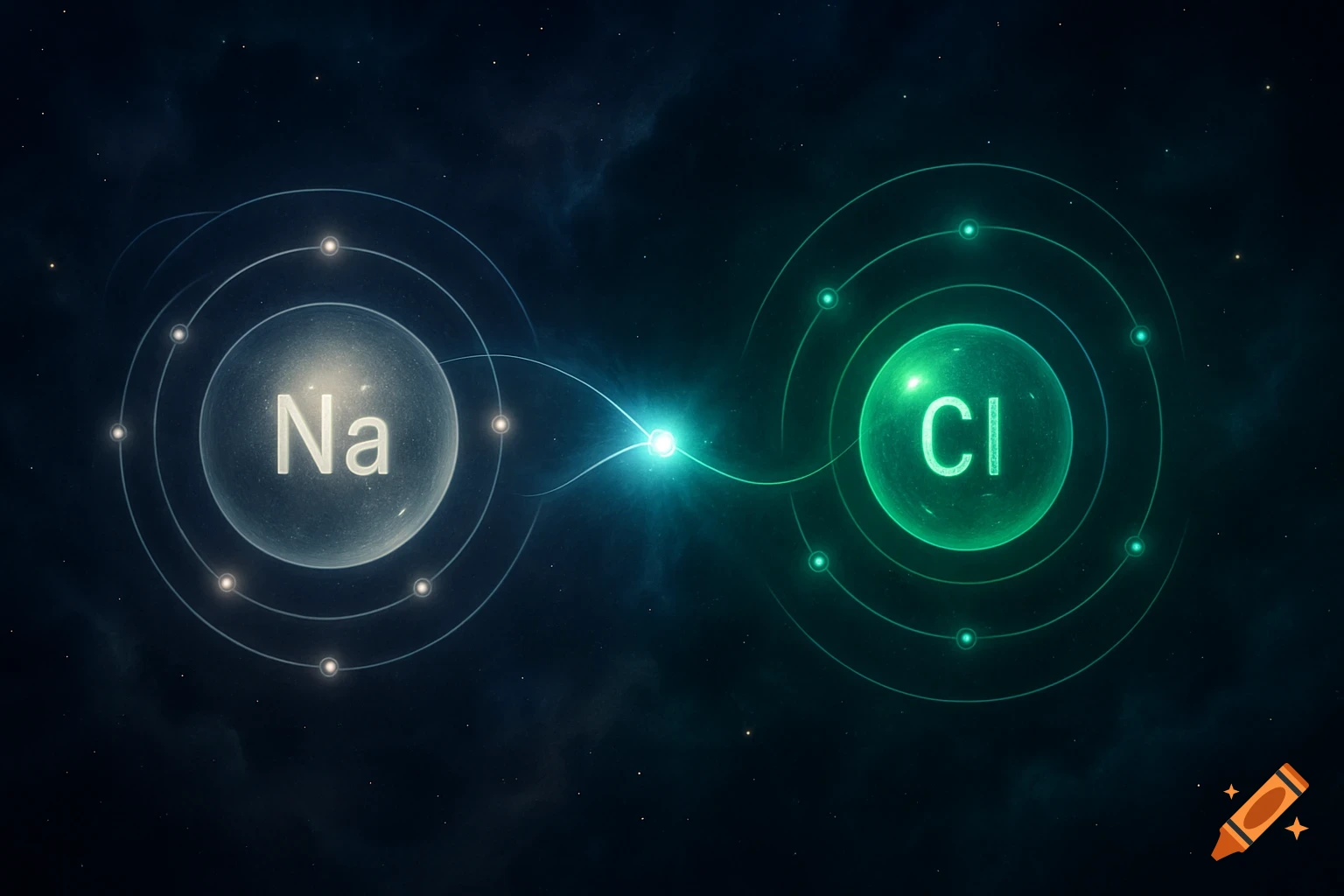
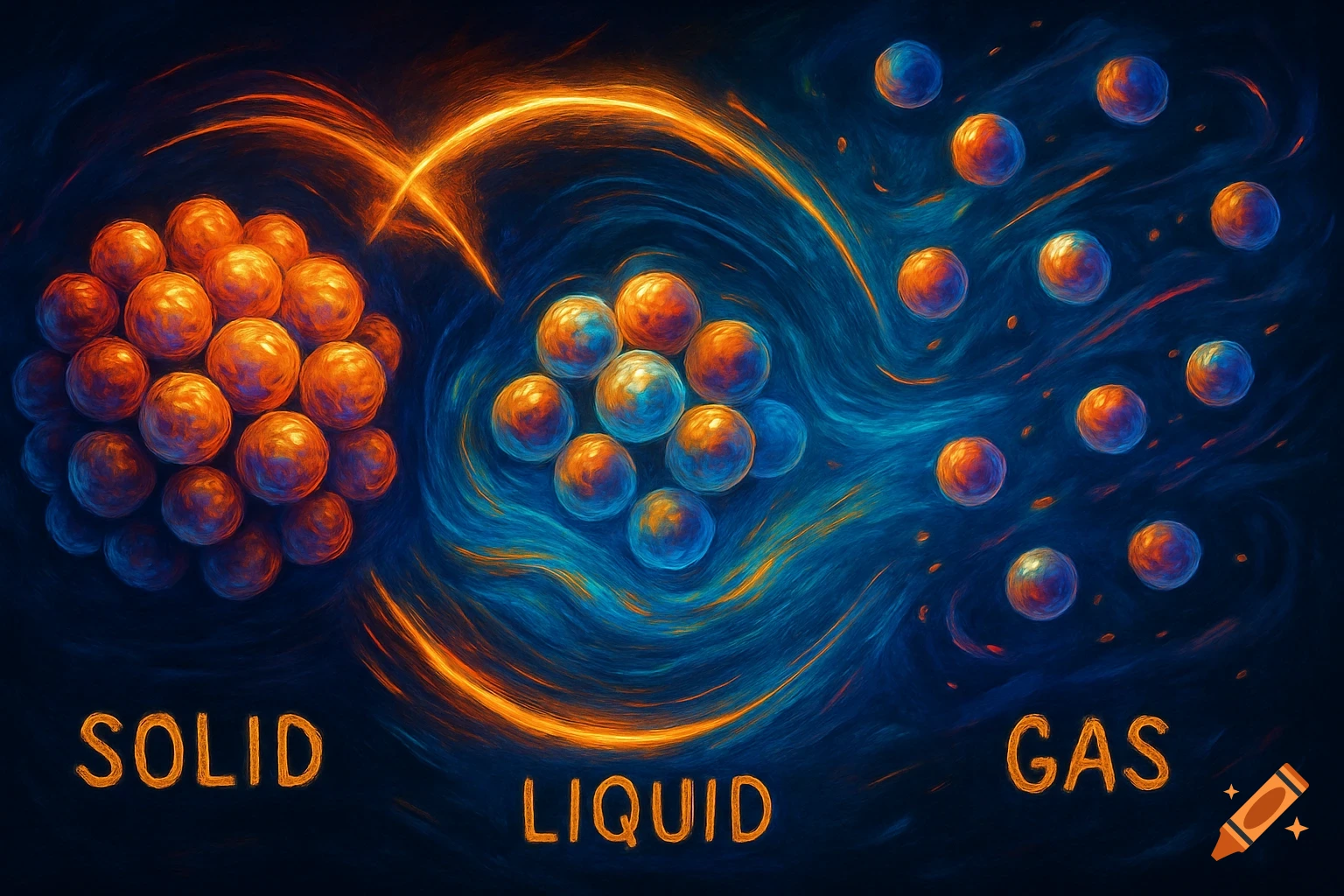
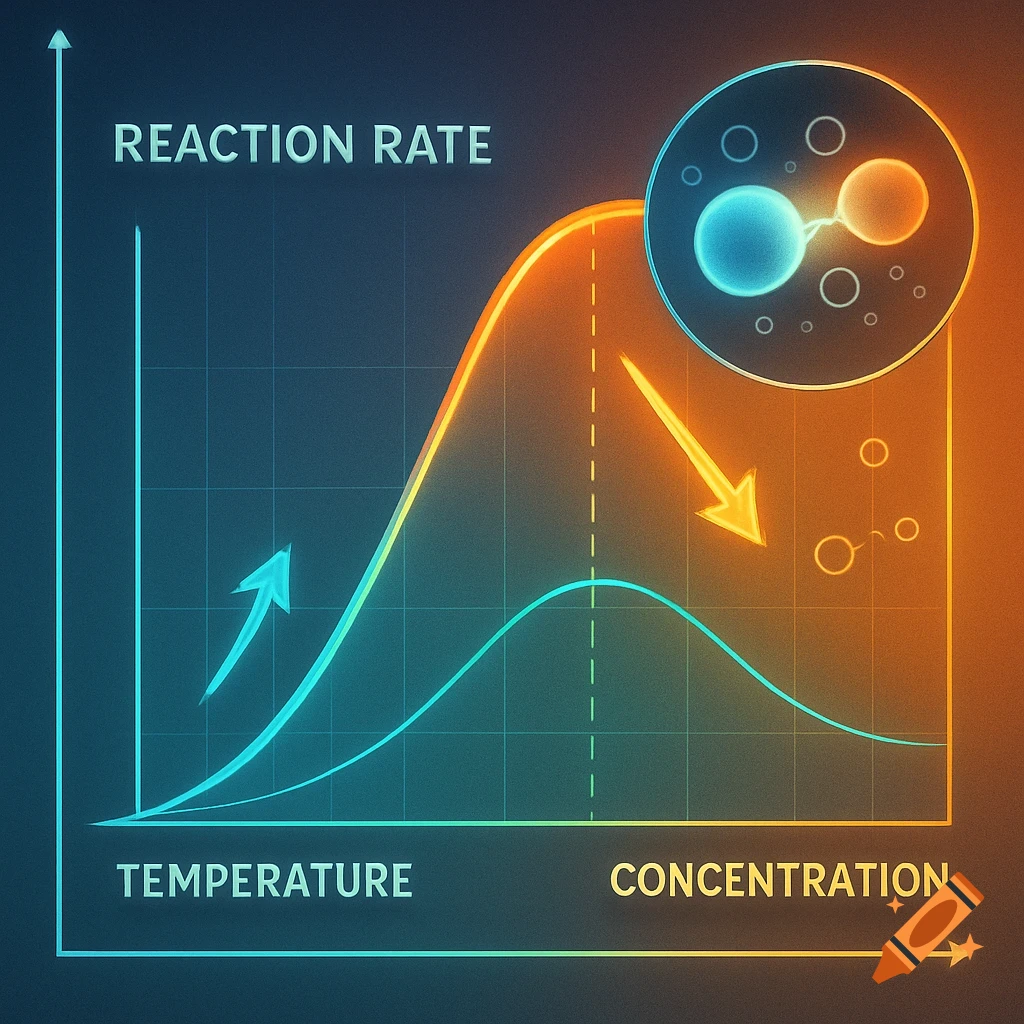
A scientific illustration of an ionic bond where a blue sodium atom (Na+) transfers an electron to a red chlorine atom (Cl-).
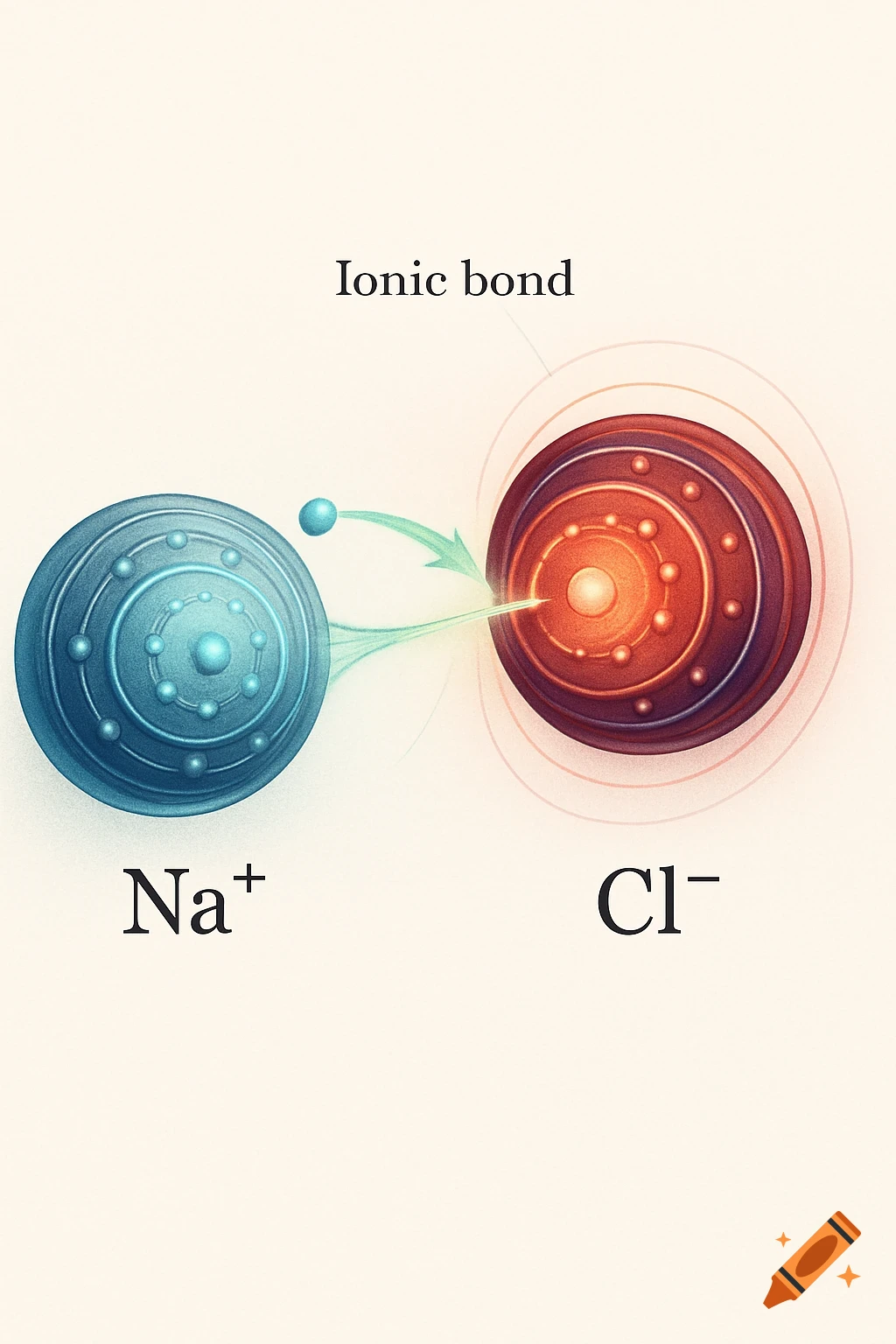
Create a detailed and scientific illustration showing the formation of an ionic bond between a sodium (Na) atom and a chlorine (Cl) atom. The sodium atom should lose one electron, becoming a positively charged ion (Na⁺), while the chlorine atom gains that electron, becoming a negatively charged ion (Cl⁻). Show the electrostatic attraction between the oppositely charged ions, highlighting the transfer of the electron and the resulting ionic bond. Include a simple background, illustrating the atoms in a 3D, molecular style, with clear labeling of the ions and their charges See more