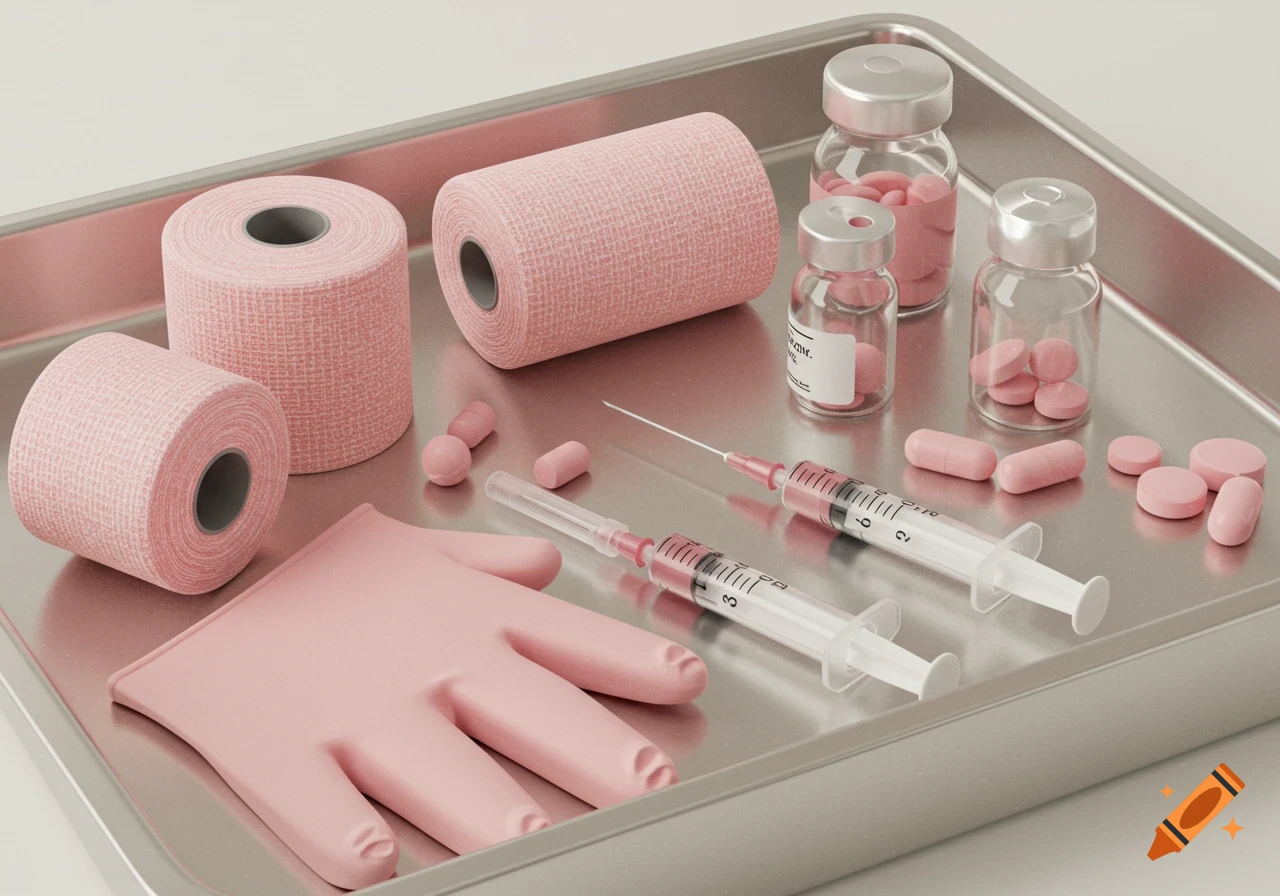
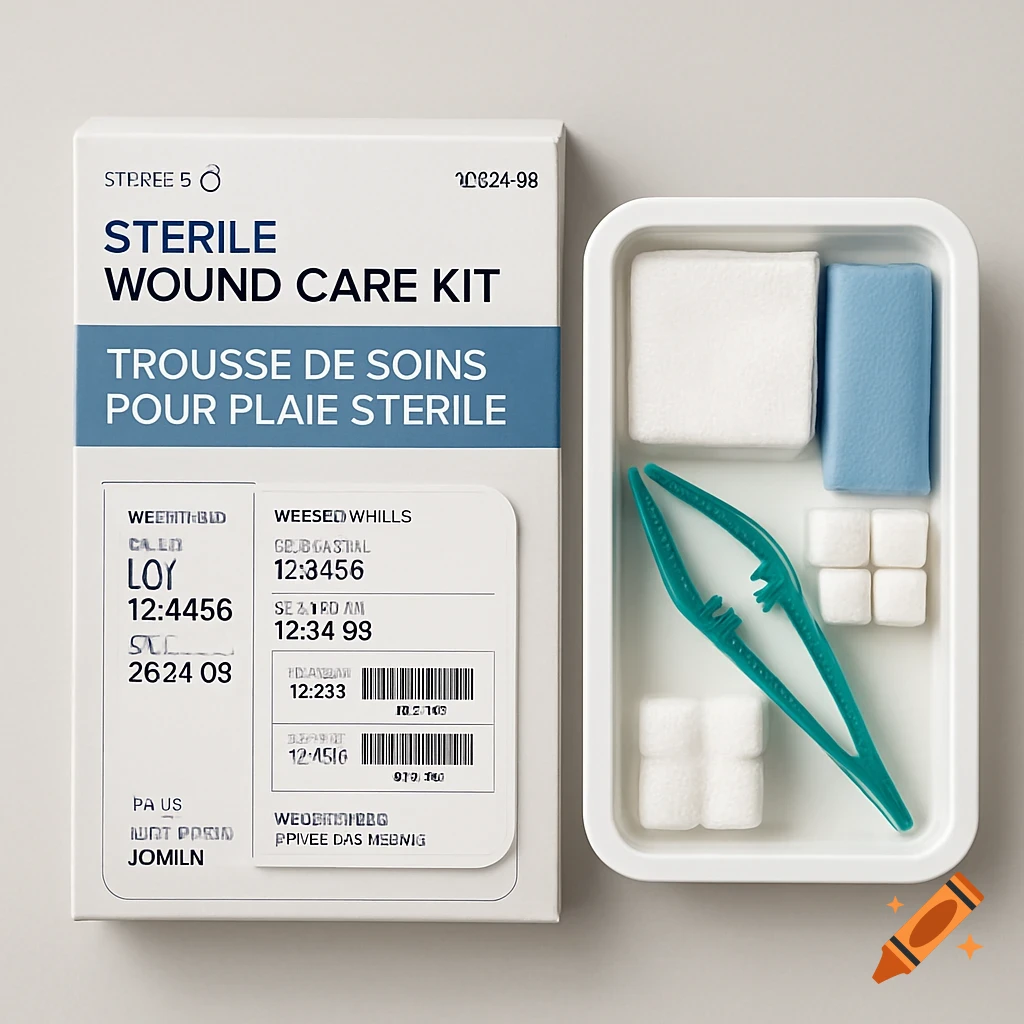
A white sterile wound care kit box with bilingual text next to an open tray containing bandages, cotton pads, and green plastic tweezers.
Description of the Invention The present invention discloses a sterile, workflow-enforcing medical device kit system engineered to optimize wound care delivery within regulated healthcare environments. The system integrates physical tray architecture, visual procedural logic, tamper-linked traceability, bilingual regulatory compliance, and subscription-based replenishment into a unified and auditable product platform. All components of the system are operatively interdependent, producing a closed-loop framework in which physical access, procedural order, and digital tracking are harmonized to reduce clinical variability and improve patient outcomes. 1. Regulatory-Compliant Outer Packaging The outer carton is constructed from rigid or semi-rigid packaging-grade materials and is designed to conform to Health Canada's Medical Device Regulations (SOR/98-282) for labeling and presentation. The carton surface includes bilingual (English/French) printed data covering device classification, lot number, expiry date, wound type, intended use, manufacturer or importer information, and the assigned Medical Device Establishment License (MDEL) number. A fold-out documentation flap or integrated pocket is included to hold scannable, removable labels that can be affixed to patient charts, inventory logs, or EMR systems. This flap may also serve as a tamper-evident surface or audit record location. The flap is pre-printed with the kit’s lot number, expiry date, and SKU in both human-readable See more